

You can:
| Name | 5-hydroxytryptamine receptor 4 |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | HTR4 |
| Synonym | 5-HT-4 5-hydroxytryptamine (serotonin) receptor 4, G protein-coupled 5-HT4 receptor 5-HT4 serotonin receptor 4 |
| Disease | N/A |
| Length | 388 |
| Amino acid sequence | MDKLDANVSSEEGFGSVEKVVLLTFLSTVILMAILGNLLVMVAVCWDRQLRKIKTNYFIVSLAFADLLVSVLVMPFGAIELVQDIWIYGEVFCLVRTSLDVLLTTASIFHLCCISLDRYYAICCQPLVYRNKMTPLRIALMLGGCWVIPTFISFLPIMQGWNNIGIIDLIEKRKFNQNSNSTYCVFMVNKPYAITCSVVAFYIPFLLMVLAYYRIYVTAKEHAHQIQMLQRAGASSESRPQSADQHSTHRMRTETKAAKTLCIIMGCFCLCWAPFFVTNIVDPFIDYTVPGQVWTAFLWLGYINSGLNPFLYAFLNKSFRRAFLIILCCDDERYRRPSILGQTVPCSTTTINGSTHVLRDAVECGGQWESQCHPPATSPLVAAQPSDT |
| UniProt | Q13639 |
| Protein Data Bank | N/A |
| GPCR-HGmod model | Q13639 |
| 3D structure model | This predicted structure model is from GPCR-EXP Q13639. |
| BioLiP | N/A |
| Therapeutic Target Database | N/A |
| ChEMBL | CHEMBL1875 |
| IUPHAR | 9 |
| DrugBank | BE0000084 |
| Name | GR 113808 |
|---|---|
| Molecular formula | C19H27N3O4S |
| IUPAC name | [1-[2-(methanesulfonamido)ethyl]piperidin-4-yl]methyl 1-methylindole-3-carboxylate |
| Molecular weight | 393.502 |
| Hydrogen bond acceptor | 6 |
| Hydrogen bond donor | 1 |
| XlogP | 1.7 |
| Synonyms | GR-113808 L000649 NCGC00015477-01 NCGC00260967-01 SCHEMBL1502039 [ Show all ] |
| Inchi Key | MOZPSIXKYJUTKI-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 |
| PubChem CID | 119376 |
| ChEMBL | CHEMBL33884 |
| IUPHAR | 247 |
| BindingDB | 29525 |
| DrugBank | N/A |
Structure | 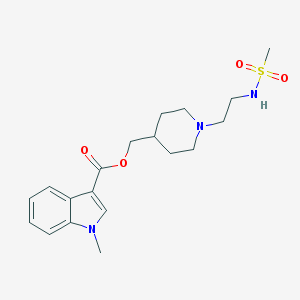 |
| Lipinski's druglikeness | This ligand satisfies Lipinski's rule of five. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| Activity | 1.0 % | PMID17676726 | ChEMBL |
| Activity | 99.0 % | PMID17676726 | ChEMBL |
| IC50 | 0.21 nM | PMID23043420 | BindingDB,ChEMBL |
| Kb | 0.03 nM | PMID23043420 | ChEMBL |
| Kd | 15.81 nM | PMID17067154 | BindingDB |
| Ki | 0.02 nM | PMID9351641 | PDSP,BindingDB |
| Ki | 0.03981 nM | PMID24113240, PMID23711770 | ChEMBL |
| Ki | 0.05 nM | PMID11218067 | BindingDB |
| Ki | 0.05 - 0.51 nM | PMID9349523, PMID9603189, PMID9351641, PMID10646498, PMID11218067, PMID11030734, PMID15351779 | IUPHAR |
| Ki | 0.07 nM | PMID9603189 | BindingDB |
| Ki | 0.07413 nM | PMID21075638 | ChEMBL |
| Ki | 0.078 nM | PMID11020291 | BindingDB,ChEMBL |
| Ki | 0.079 nM | PMID11218067 | BindingDB |
| Ki | 0.09 nM | PMID9351641 | PDSP,BindingDB |
| Ki | 0.1 nM | PMID11020291 | BindingDB,ChEMBL |
| Ki | 0.15 nM | PMID9349523, PMID8867105 | PDSP,BindingDB |
| Ki | 0.1585 nM | PMID19261477 | ChEMBL |
| Ki | 0.25 nM | PMID10646498, PMID9349523 | PDSP,BindingDB |
| Ki | 0.251189 nM | PMID25149506 | BindingDB |
| Ki | 0.2512 nM | PMID25149506 | ChEMBL |
| Ki | 0.31 nM | PMID10646498 | BindingDB |
| Ki | 0.33 nM | PMID11020291, PMID9603189 | BindingDB,ChEMBL |
| Ki | 0.41 nM | PMID11020291, PMID9603189 | BindingDB,ChEMBL |
| Ki | 0.5 nM | PMID10646498, PMID17676726 | BindingDB,ChEMBL |
| Ki | 0.53 nM | PMID11020291, PMID9603189 | BindingDB,ChEMBL |
| Ki | 0.7 nM | PMID17676726 | BindingDB,ChEMBL |
| pKb | 8.7 - | PMID24113240, PMID23711770 | ChEMBL |
| pKb | 9.24 - | PMID21075638 | ChEMBL |
| pKb | 10.1 - | PMID25149506 | ChEMBL |
zhanglab![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218