

You can:
| Name | Histamine H1 receptor |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | HRH1 |
| Synonym | HH1R H1R Hisr H1 receptor |
| Disease | Vertigo's disease; Meniere's disease Ocular allergy Obesity Nausea; Vomiting Insomnia; Anxiety disorder [ Show all ] |
| Length | 487 |
| Amino acid sequence | MSLPNSSCLLEDKMCEGNKTTMASPQLMPLVVVLSTICLVTVGLNLLVLYAVRSERKLHTVGNLYIVSLSVADLIVGAVVMPMNILYLLMSKWSLGRPLCLFWLSMDYVASTASIFSVFILCIDRYRSVQQPLRYLKYRTKTRASATILGAWFLSFLWVIPILGWNHFMQQTSVRREDKCETDFYDVTWFKVMTAIINFYLPTLLMLWFYAKIYKAVRQHCQHRELINRSLPSFSEIKLRPENPKGDAKKPGKESPWEVLKRKPKDAGGGSVLKSPSQTPKEMKSPVVFSQEDDREVDKLYCFPLDIVHMQAAAEGSSRDYVAVNRSHGQLKTDEQGLNTHGASEISEDQMLGDSQSFSRTDSDTTTETAPGKGKLRSGSNTGLDYIKFTWKRLRSHSRQYVSGLHMNRERKAAKQLGFIMAAFILCWIPYFIFFMVIAFCKNCCNEHLHMFTIWLGYINSTLNPLIYPLCNENFKKTFKRILHIRS |
| UniProt | P35367 |
| Protein Data Bank | 3rze |
| GPCR-HGmod model | P35367 |
| 3D structure model | This structure is from PDB ID 3rze. |
| BioLiP | BL0202178, BL0202179, BL0202180 |
| Therapeutic Target Database | T77913 |
| ChEMBL | CHEMBL231 |
| IUPHAR | 262 |
| DrugBank | BE0000442 |
| Name | histamine |
|---|---|
| Molecular formula | C5H9N3 |
| IUPAC name | 2-(1H-imidazol-5-yl)ethanamine |
| Molecular weight | 111.148 |
| Hydrogen bond acceptor | 2 |
| Hydrogen bond donor | 2 |
| XlogP | -0.7 |
| Synonyms | 4-Imidazoleethylamine 820484N8I3 2-(1H-Imidazol-4-yl)-ethylamine AJ-70500 2-(3H-imidazol-4-yl)ethanamine [ Show all ] |
| Inchi Key | NTYJJOPFIAHURM-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) |
| PubChem CID | 774 |
| ChEMBL | CHEMBL90 |
| IUPHAR | 1247, 1204 |
| BindingDB | 7966, 50121205 |
| DrugBank | DB05381 |
Structure | 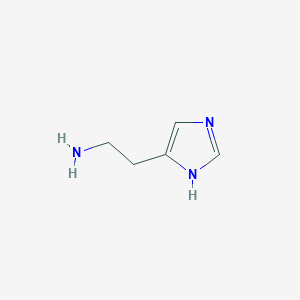 |
| Lipinski's druglikeness | This ligand satisfies Lipinski's rule of five. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| N/A | N/A | DrugBank | |
| Alpha | 1.0 - | PMID14667234 | ChEMBL |
| Change | 284.0 % | PMID2959777 | ChEMBL |
| EC50 | 13.0 nM | PMID12723960 | BindingDB,ChEMBL |
| EC50 | 45.0 nM | PMID16942032 | BindingDB,ChEMBL |
| EC50 | 190.0 nM | PMID18950149, PMID27007611 | BindingDB,ChEMBL |
| EC50 | 190.55 nM | PMID19317445, MedChemComm, (2014) 5:1:72, PMID20409707, PMID19791743 | BindingDB,ChEMBL |
| EC50 | 191.0 nM | PMID19317445 | BindingDB |
| EC50 | 199.53 nM | PMID21044842 | ChEMBL |
| Emax | 1.0 % | PMID20409707 | ChEMBL |
| IC50 | 158.0 nM | PMID14667234 | BindingDB |
| IC50 | 158.49 nM | PMID14667234 | ChEMBL |
| IC50 | 1000.0 nM | Bioorg. Med. Chem. Lett., (1997) 7:22:2819 | ChEMBL |
| Ki | <10000.0 nM | http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?CMD=search&DB=pubmed, PMID8335064, PMID6146381, PMID12065734 | PDSP,BindingDB |
| Ki | 190.0 nM | PMID16554355 | PDSP,BindingDB |
| Ki | 1258.92 nM | PMID8903934, PMID7925364 | PDSP,BindingDB |
| Ki | 1258.93 nM | PMID11809864 | PDSP |
| Ki | 1258.93 - 19952.6 nM | PMID7925364, PMID12065734, PMID16394198, PMID12626648, PMID15206929 | IUPHAR |
| Ki | 1258.93 nM | PMID11809864 | BindingDB |
| Ki | 2000.0 nM | PMID16554355 | PDSP |
| Ki | 2060.0 nM | PMID12626648 | PDSP,BindingDB |
| Ki | 6000.0 nM | PMID25993395 | BindingDB,ChEMBL |
| Ki | 7010.0 nM | PMID15033391 | PDSP,BindingDB |
| Ki | 79432.8 nM | PMID16408006 | BindingDB,ChEMBL |
| Ki | 1.58489e+13 nM | PMID14667234 | ChEMBL |
zhanglab![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218