

You can:
| Name | D(1A) dopamine receptor |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | DRD1 |
| Synonym | D1 receptor D1A DADR Gpcr15 dopamine D1 receptor [ Show all ] |
| Disease | Unspecified Hypertension Pain Parkinson's disease Psychiatric disorder [ Show all ] |
| Length | 446 |
| Amino acid sequence | MRTLNTSAMDGTGLVVERDFSVRILTACFLSLLILSTLLGNTLVCAAVIRFRHLRSKVTNFFVISLAVSDLLVAVLVMPWKAVAEIAGFWPFGSFCNIWVAFDIMCSTASILNLCVISVDRYWAISSPFRYERKMTPKAAFILISVAWTLSVLISFIPVQLSWHKAKPTSPSDGNATSLAETIDNCDSSLSRTYAISSSVISFYIPVAIMIVTYTRIYRIAQKQIRRIAALERAAVHAKNCQTTTGNGKPVECSQPESSFKMSFKRETKVLKTLSVIMGVFVCCWLPFFILNCILPFCGSGETQPFCIDSNTFDVFVWFGWANSSLNPIIYAFNADFRKAFSTLLGCYRLCPATNNAIETVSINNNGAAMFSSHHEPRGSISKECNLVYLIPHAVGSSEDLKKEEAAGIARPLEKLSPALSVILDYDTDVSLEKIQPITQNGQHPT |
| UniProt | P21728 |
| Protein Data Bank | N/A |
| GPCR-HGmod model | P21728 |
| 3D structure model | This predicted structure model is from GPCR-EXP P21728. |
| BioLiP | N/A |
| Therapeutic Target Database | T22118 |
| ChEMBL | CHEMBL2056 |
| IUPHAR | 214 |
| DrugBank | BE0000020 |
| Name | UNII-UGT5535REQ |
|---|---|
| Molecular formula | C17H18ClNO |
| IUPAC name | (5R)-8-chloro-3-methyl-5-phenyl-1,2,4,5-tetrahydro-3-benzazepin-7-ol |
| Molecular weight | 287.787 |
| Hydrogen bond acceptor | 2 |
| Hydrogen bond donor | 1 |
| XlogP | 4.0 |
| Synonyms | 87075-17-0 CCG-204548 NCGC00024877-02 Sch 23388 UGT5535REQ [ Show all ] |
| Inchi Key | GOTMKOSCLKVOGG-OAHLLOKOSA-N |
| Inchi ID | InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 |
| PubChem CID | 3036864 |
| ChEMBL | CHEMBL62 |
| IUPHAR | N/A |
| BindingDB | 82247 |
| DrugBank | N/A |
Structure | 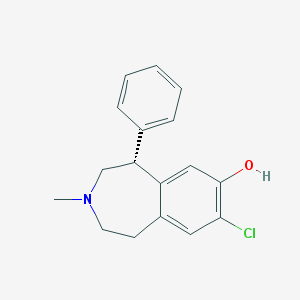 |
| Lipinski's druglikeness | This ligand satisfies Lipinski's rule of five. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| Activity | 100.0 % | PMID18562201 | ChEMBL |
| IC50 | 0.74 nM | PMID18588282 | ChEMBL |
| IC50 | 0.82 nM | PMID20875743 | BindingDB |
| IC50 | 0.82 nM | PMID20875743 | ChEMBL |
| IC50 | 0.96 nM | PMID18983139 | ChEMBL |
| IC50 | 1.4 nM | PMID23403082 | ChEMBL |
| IC50 | 1.7 nM | PMID24805037 | BindingDB |
| IC50 | 2.52 nM | PMID22748706 | BindingDB,ChEMBL |
| IC50 | 30.0 nM | PMID25308766 | BindingDB |
| IC50 | 520.0 nM | PMID22748706 | BindingDB,ChEMBL |
| IC50 | 600.0 nM | PMID23332346 | BindingDB |
| Imax | 80.05 % | PMID22748706 | ChEMBL |
| Inhibition | -12.0 % | PMID23403082 | ChEMBL |
| Kd | 0.38 nM | PMID3263503 | BindingDB,ChEMBL |
| Ki | 0.1 nM | PMID25557493, PMID24805037 | BindingDB |
| Ki | 0.11 nM | PMID21726069 | BindingDB,ChEMBL |
| Ki | 0.15 nM | PMID2405157 | BindingDB |
| Ki | 0.15 nM | PMID2405157 | BindingDB,ChEMBL |
| Ki | 0.17 nM | PMID1531075, PMID1831904 | BindingDB |
| Ki | 0.3 nM | PMID1531365, PMID1973733 | ChEMBL |
| Ki | 0.3 nM | PMID1531365, PMID1973733 | BindingDB |
| Ki | 0.35 nM | PMID1826762 | BindingDB |
| Ki | 0.38 nM | PMID18983139 | ChEMBL |
| Ki | 0.4266 nM | PMID2527994 | ChEMBL |
| Ki | 0.7 nM | PMID23403082 | ChEMBL |
| Ki | 0.8 nM | PMID9686407, PMID18562201 | BindingDB,ChEMBL |
| Ki | 0.94 nM | PMID23018094 | BindingDB |
| Ki | 1.0 nM | PMID19643610 | BindingDB |
| Ki | 1.2 nM | PMID23332346 | BindingDB |
| Ki | 1.24 nM | PMID22748706 | BindingDB,ChEMBL |
| Ki | 1.4 nM | PMID20061148 | BindingDB,ChEMBL |
| Ki | 1.69 nM | PMID26227779 | ChEMBL |
| Ki | 1.7 nM | PMID26227779 | BindingDB |
| Ki | 15.0 nM | PMID1831904 | BindingDB |
| Ki | 24.0 nM | PMID2405157 | ChEMBL |
| Ki | 41.0 nM | PMID1826762 | BindingDB |
| nH | 1.05 - | PMID8558526 | ChEMBL |
| Potency | 3.7 nM | PubChem BioAssay data set | ChEMBL |
| Potency | 14.6 nM | PubChem BioAssay data set | ChEMBL |
| Potency | 1584890.0 nM | PubChem BioAssay data set | ChEMBL |
zhanglab![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218