

You can:
| Name | Alpha-1A adrenergic receptor |
|---|---|
| Species | Rattus norvegicus (Rat) |
| Gene | Adra1a |
| Synonym | alpha1c alpha1A-adrenoceptor alpha1a Alpha-1C adrenergic receptor Alpha-1A adrenoreceptor [ Show all ] |
| Disease | N/A for non-human GPCRs |
| Length | 466 |
| Amino acid sequence | MVLLSENASEGSNCTHPPAPVNISKAILLGVILGGLIIFGVLGNILVILSVACHRHLHSVTHYYIVNLAVADLLLTSTVLPFSAIFEILGYWAFGRVFCNIWAAVDVLCCTASIMGLCIISIDRYIGVSYPLRYPTIVTQRRGVRALLCVWVLSLVISIGPLFGWRQPAPEDETICQINEEPGYVLFSALGSFYVPLAIILVMYCRVYVVAKRESRGLKSGLKTDKSDSEQVTLRIHRKNVPAEGGGVSSAKNKTHFSVRLLKFSREKKAAKTLGIVVGCFVLCWLPFFLVMPIGSFFPDFKPSETVFKIVFWLGYLNSCINPIIYPCSSQEFKKAFQNVLRIQCLRRRQSSKHALGYTLHPPSQALEGQHRDMVRIPVGSGETFYKISKTDGVCEWKFFSSMPQGSARITVPKDQSACTTARVRSKSFLQVCCCVGSSAPRPEENHQVPTIKIHTISLGENGEEV |
| UniProt | P43140 |
| Protein Data Bank | N/A |
| GPCR-HGmod model | N/A |
| 3D structure model | No available structures or models |
| BioLiP | N/A |
| Therapeutic Target Database | N/A |
| ChEMBL | CHEMBL319 |
| IUPHAR | 22 |
| DrugBank | N/A |
| Name | prazosin |
|---|---|
| Molecular formula | C19H21N5O4 |
| IUPAC name | [4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl]-(furan-2-yl)methanone |
| Molecular weight | 383.408 |
| Hydrogen bond acceptor | 8 |
| Hydrogen bond donor | 1 |
| XlogP | 2.0 |
| Synonyms | Hypovase (TN) Pressin 2-(4-(2-FUROYL)-1-PIPERAZINYL)-6,7-DIMETHOXY-4-QUINAZOLINAMINE KBio2_001302 SBI-0051487.P003 [ Show all ] |
| Inchi Key | IENZQIKPVFGBNW-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) |
| PubChem CID | 4893 |
| ChEMBL | CHEMBL2 |
| IUPHAR | 503 |
| BindingDB | 29568 |
| DrugBank | DB00457 |
Structure | 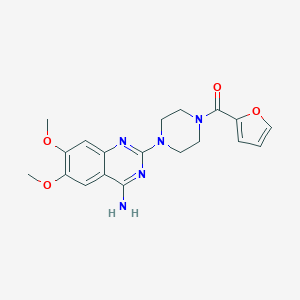 |
| Lipinski's druglikeness | This ligand satisfies Lipinski's rule of five. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| IC50 | 0.218 nM | DrugMatrix in vitro pharmacology data | ChEMBL |
| IC50 | 0.254 nM | PMID19788200 | BindingDB |
| IC50 | 0.69 nM | PMID23403082 | ChEMBL |
| Inhibition | 5.0 % | PMID23403082 | ChEMBL |
| Kd | 0.1549 nM | PMID8523408 | ChEMBL |
| Kd | 0.155 nM | PMID8523408 | BindingDB |
| Kd | 1.047 nM | PMID15633998 | ChEMBL |
| Kd | 1.05 nM | PMID15633998 | BindingDB |
| Kd | 2.51 nM | PMID9822553 | BindingDB |
| Kd | 2.512 nM | PMID9871765, PMID9822553 | ChEMBL |
| Ki | <10000.0 nM | PMID8386236 | BindingDB |
| Ki | 0.01 nM | PMID2885412 | BindingDB |
| Ki | 0.02 nM | PMID2885412 | BindingDB |
| Ki | 0.07 nM | PMID7815325, PMID10602703 | BindingDB,ChEMBL |
| Ki | 0.088 nM | DrugMatrix in vitro pharmacology data | ChEMBL |
| Ki | 0.12 nM | PMID10611634 | BindingDB |
| Ki | 0.23 nM | PMID16723224 | BindingDB,ChEMBL |
| Ki | 0.28 nM | PMID23403082 | ChEMBL |
| Ki | 0.3 nM | PMID9548811 | BindingDB,ChEMBL |
| Ki | 0.31 nM | PMID6144048 | BindingDB |
| Ki | 0.316228 nM | PMID11331292 | IUPHAR |
| Ki | 0.33 nM | PMID1706716 | BindingDB |
| Ki | 0.9333 nM | PMID8917649 | ChEMBL |
| Ki | 1.05 nM | PMID9572880 | BindingDB,ChEMBL |
| Ki | 5710.0 nM | PMID2531826 | BindingDB |
| pKB | 8.93 - | PMID14584940 | ChEMBL |
| pKb | 8.99 - | PMID11462977 | ChEMBL |
zhanglab![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218