

You can:
| Name | Pituitary adenylate cyclase-activating polypeptide type I receptor |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | ADCYAP1R1 |
| Synonym | PACAPR1 pituitary adenylate cyclase activating polypeptide 1 receptor (1) PACAP1-R PACAP-R1 PACAP-R-1 [ Show all ] |
| Disease | N/A |
| Length | 468 |
| Amino acid sequence | MAGVVHVSLAALLLLPMAPAMHSDCIFKKEQAMCLEKIQRANELMGFNDSSPGCPGMWDNITCWKPAHVGEMVLVSCPELFRIFNPDQVWETETIGESDFGDSNSLDLSDMGVVSRNCTEDGWSEPFPHYFDACGFDEYESETGDQDYYYLSVKALYTVGYSTSLVTLTTAMVILCRFRKLHCTRNFIHMNLFVSFMLRAISVFIKDWILYAEQDSNHCFISTVECKAVMVFFHYCVVSNYFWLFIEGLYLFTLLVETFFPERRYFYWYTIIGWGTPTVCVTVWATLRLYFDDTGCWDMNDSTALWWVIKGPVVGSIMVNFVLFIGIIVILVQKLQSPDMGGNESSIYLRLARSTLLLIPLFGIHYTVFAFSPENVSKRERLVFELGLGSFQGFVVAVLYCFLNGEVQAEIKRKWRSWKVNRYFAVDFKHRHPSLASSGVNGGTQLSILSKSSSQIRMSGLPADNLAT |
| UniProt | P41586 |
| Protein Data Bank | 3n94 |
| GPCR-HGmod model | P41586 |
| 3D structure model | This structure is from PDB ID 3n94. |
| BioLiP | BL0183307 |
| Therapeutic Target Database | N/A |
| ChEMBL | CHEMBL5399 |
| IUPHAR | 370 |
| DrugBank | N/A |
| Name | CHEMBL153038 |
|---|---|
| Molecular formula | C26H24ClN3O2 |
| IUPAC name | 3-chloro-4-hydroxy-N-[(E)-[1-[(4-propan-2-ylphenyl)methyl]indol-4-yl]methylideneamino]benzamide |
| Molecular weight | 445.947 |
| Hydrogen bond acceptor | 3 |
| Hydrogen bond donor | 2 |
| XlogP | 5.9 |
| Synonyms | 3-Chloro-4-hydroxy-benzoic acid [1-(4-isopropyl-benzyl)-1H-indol-4-ylmethylene]-hydrazide BDBM50110055 N''-((1-(4-isopropylbenzyl)-1H-indol-4-yl)methylene)-3-chloro-4-hydroxybenzohydrazide 3-Chloro-4-hydroxy-benzoic acid [1-[1-(4-isopropyl-benzyl)-1H-indol-4-yl]-meth-(E)-ylidene]-hydrazide |
| Inchi Key | JJGVEWLQTOEULJ-RWPZCVJISA-N |
| Inchi ID | InChI=1S/C26H24ClN3O2/c1-17(2)19-8-6-18(7-9-19)16-30-13-12-22-21(4-3-5-24(22)30)15-28-29-26(32)20-10-11-25(31)23(27)14-20/h3-15,17,31H,16H2,1-2H3,(H,29,32)/b28-15+ |
| PubChem CID | 9824797 |
| ChEMBL | CHEMBL153038 |
| IUPHAR | N/A |
| BindingDB | 50110055 |
| DrugBank | N/A |
Structure | 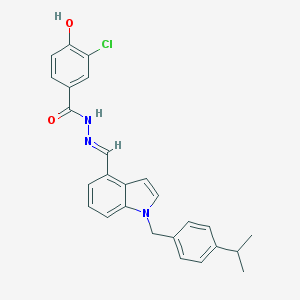 |
| Lipinski's druglikeness | This ligand has a partition coefficient log P greater than 5. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| Ki | 604.0 nM | PMID18272364 | BindingDB,ChEMBL |
zhanglab![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218