| Synonyms | R 55,667
BSPBio_002805
Ritanserin [USAN:INN:BAN]
CHEMBL267777
SBI-0051053.P003
DivK1c_000192
FT-0630948
I14-31889
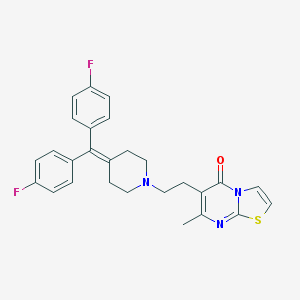
5h-thiazolo[3,2-a]pyrimidin-5-one, 6-[2-[4-[bis(4-fluorophenyl)methylene]-1-piperidinyl]ethyl]-7-methyl-
KBioSS_002335
6-(2-{4-[bis(4-fluorophenyl)methylidene]piperidin-1-yl}ethyl)-7-methyl-5H-[1,3]thiazolo[3,2-a]pyrimidin-5-one
MLS000069360
6-[2-[4-[bis(4-fluorophenyl)-methylene]-1-piperidinyl]-ethyl]-5h-thiazolo[3,2-a]pyrimidin-5-one
NCGC00015877-05
87051-43-2
NCGC00022447-05
API0004083
Tox21_110251_1
REGID_for_CID_5074
CAS-87051-43-2
Ritanserina [Spanish]
D05738
SPBio_001440
DSSTox_RID_80055
HMS2233M22
3726AC
KBio1_000192
6-(2-(4-(Bis(4-fluorophenyl)methylene)-1-piperidinyl)ethyl)-7-methyl-5H-thiazolo((3,2-a)pyrimidin-5-one
Lopac-R-103
6-(2-{4-[Bis-(4-fluoro-phenyl)-methylene]-piperidin-1-yl}-ethyl)-7-methyl-thiazolo[3,2-a]pyrimidin-5-one(Ritanserin)
N,N-dialkyl-dipeptidylamines
6-[2-[4-[bis(4-fluorophenyl)methylene]-1-piperidyl]ethyl]-7-methyl-thiazolo[3,2-a]pyrimidin-5-one
NCGC00015877-08
AC1L1JJX
NINDS_000192
BCP13728
SR-01000000024-3
ZINC538314
BRD-K40887525-001-02-9
ritanserin serotonin antagonist
CHEBI:64195
Ritanserinum
Spectrum5_001504
ethyl]-7-methyl-5H-thiazolo[3,2-a]pyrimidin-5-one
(ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methylene]-piperidin-1-yl}-ethyl)-7-methyl-2,3-dihydro-1H-indolizin-5-one
HMS3373O19
5H-Thiazolo(3,2-a)pyrimidin-5-one, 6-(2-(4-bis(4-fluorophenyl)methylene)-1-piperidinyl)ethyl)-7-methyl-
KBio2_007468
6-(2-(4-(Bis(p-fluorophenyl)methylene)-piperidino)ethyl)-7-methyl-5H-thiazolo-(3,2-a)pyrimidin-5-one
LS-156826
6-[2-[4-[bis (4-Fluorophenyl)- methylene]-1-piperidinyl]-ethyl]- 5H-thiazolo[3,2-a]pyrimidin-5-one
NCGC00015877-03
6-[2-[4-[bis(4-fluorophenyl)methylidene]piperidin-1-yl]ethyl]-7-methyl-[1,3]thiazolo[3,2-a]pyrimidin-5-one
NCGC00022447-03
AKOS015909799
Opera_ID_1609
Tiserton (TN)
R-103
C-22775
Ritanserin, powder
cid_5074
SCHEMBL49227
DSSTox_CID_22594
GTPL97
051R432
IDI1_000192
5H-Thiazolo[3,2-a]pyrimidin-5-one,6-[2-[4-[bis(4-fluorophenyl)methylene]-1-piperidinyl]ethyl]-7-methyl-
KS-00001841
6-(2-{4-[Bis-(4-fluoro-phenyl)-methylene]-piperidin-1-yl}-ethyl)-7-methyl-thiazolo[3,2-a]pyrimidin-5-one
MLS001148629
6-[2-[4-[bis(4-fluorophenyl)-methylene]-1-piperidinyl]ethyl]-7-methyl-5H-thiazolo[3,2-a]-pyrimidin-5-one
NCGC00015877-06
NCGC00178460-01
AX8150457
Tox21_501083
CC-34269
Ritanserine
D0K8NW
Spectrum2_001560
DTXSID9042594
HMS3263I08
KBio2_002332
6-(2-(4-(Bis(4-fluorophenyl)methylene)piperidin-1-yl)-ethyl)-7-methyl-5H-thiazolo[3,2-a]pyrimidin-5-one
Lopac0_001083
6-[2-[4-(bis(4-Fluorophenyl)methylene]-1-piperidinyl]ethyl]-7-methyl-5H-thiazolo[3,2-a]pyrimidin-5-one
NCGC00015877-01
6-[2-[4-[Bis(4-fluorophenyl)Methylidene]piperidin-1-yl]ethyl]-7-Methyl-[1,3]thia
NCGC00015877-09
AC1Q4NH0
NSC-758470
BDBM50001775
SR-01000000024-4
BRD-K40887525-001-14-4
Ritanserin [USAN:BAN:INN]
Ritanserinum [Latin]
DB12693
Spectrum_001830
EU-0101083
(ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methylene]-piperidin-1-yl}-ethyl)-7-methyl-thiazolo[3,2-a]pyrimidin-5-one
HMS500J14
5H-Thiazolo[3,2-a]pyrimidin-5-one, 6-[2-[4-bis(4-fluorophenyl)methylene]-1-piperidinyl]ethyl]-7-methyl-
KBio3_002025
6-(2-(4-[Bis(4-fluorophenyl)methylene]-1-piperidinyl)ethyl)-7-methyl-5H-[1,3]thiazolo[3,2-a]pyrimidin-5-one #
MFCD00069341
6-[2-[4-[bis (4-Fluorophenyl)-methylene]-1-piperidinyl]-ethyl]-5H-thiazolo[3,2-a]pyrimidin-5-one
NCGC00015877-04
6-[2-[4-[Bis(p-fluorophenyl)methylene]-piperidino]ethyl]-7-methyl-5H-thiazolo-[3,2-a]pyrimidin-5-one
NCGC00022447-04
AN-16598
Pharmakon1600-01503421
Tox21_110251
R-55667
C27H25F2N3OS
Ritanserina
CTK5F7657
SMR000058511
DSSTox_GSID_42594
HMS2093E19
145TFV465S
JUQLTPCYUFPYKE-UHFFFAOYSA-N
6-(2-(4-(Bis(4-fluorophenyl);methylene);piperidin-1-yl);ethyl);-7-methyl-5H-thiazolo[3,2-a]pyrimidin-5-one
L001003
6-(2-{4-[Bis-(4-fluoro-phenyl)-methylene]-piperidin-1-yl}-ethyl)-7-methyl-thiazolo[3,2-a]pyrimidin-5-one (Ritanserin)
MolPort-003-666-486
6-[2-[4-[bis(4-fluorophenyl)methylene]-1-piperidinyl]ethyl]-7-methyl-5H-thiazolo[3,2-a]pyrimidin-5-one
NCGC00015877-07
AB00053288_14
NCGC00261768-01
B6898
SR-01000000024
UNII-145TFV465S
BG0387
Ritanserin (USAN/INN)
CCG-39338
Ritanserine [French]
D0N2OM
Spectrum3_001023
E2J
(+)-6-[2-[4-[bis(4-fluorophenyl)methylene]-1-piperidinyl]ethyl]-7-methyl-5H-thiazolo[3,2-a]pyrimidin-5-one
HMS3268O04
5H-Thiazolo(3,2-a)pyrimidin-5-one, 6-(2-(4-(bis(4-fluorophenyl)methylene)-1-piperidinyl)ethyl)-7-methyl-
KBio2_004900
6-(2-(4-(bis(4-fluorophenyl)methylene)piperidin-1-yl)ethyl)-7-methyl-5H-thiazolo[3,2-a]pyrimidin-5-one
LP01083
6-[2-[4-bis(4-Fluorophenyl)methylene]-1-piperidinyl]-
NCGC00015877-02
6-[2-[4-[bis(4-fluorophenyl)methylidene]piperidin-1-yl]ethyl]-7-methyl-[1,3]thiazolo[2,3-b]pyrimidin-5-one
NCGC00015877-11
AJ-23387
NSC758470
Tiserton [ Show all ] |
|---|


![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218