

You can:
| Name | Cannabinoid receptor 1 |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | CNR1 |
| Synonym | CB1 Central cannabinoid receptor SKR6R THC receptor CB1R [ Show all ] |
| Disease | Obesity; Diabetes Chemotherapy-induced nausea Diabetes; Obesity Drug abuse Hypertension; Diabetes; Obesity [ Show all ] |
| Length | 472 |
| Amino acid sequence | MKSILDGLADTTFRTITTDLLYVGSNDIQYEDIKGDMASKLGYFPQKFPLTSFRGSPFQEKMTAGDNPQLVPADQVNITEFYNKSLSSFKENEENIQCGENFMDIECFMVLNPSQQLAIAVLSLTLGTFTVLENLLVLCVILHSRSLRCRPSYHFIGSLAVADLLGSVIFVYSFIDFHVFHRKDSRNVFLFKLGGVTASFTASVGSLFLTAIDRYISIHRPLAYKRIVTRPKAVVAFCLMWTIAIVIAVLPLLGWNCEKLQSVCSDIFPHIDETYLMFWIGVTSVLLLFIVYAYMYILWKAHSHAVRMIQRGTQKSIIIHTSEDGKVQVTRPDQARMDIRLAKTLVLILVVLIICWGPLLAIMVYDVFGKMNKLIKTVFAFCSMLCLLNSTVNPIIYALRSKDLRHAFRSMFPSCEGTAQPLDNSMGDSDCLHKHANNAASVHRAAESCIKSTVKIAKVTMSVSTDTSAEAL |
| UniProt | P21554 |
| Protein Data Bank | 5tjv, 5u09, 5xr8, 5xra, 6n4b, 5tgz |
| GPCR-HGmod model | P21554 |
| 3D structure model | This structure is from PDB ID 5tjv. |
| BioLiP | BL0384680, BL0364157, BL0384679, BL0384681, BL0384682, BL0384683, BL0384684, BL0440253, BL0440254,BL0440255, BL0363267, BL0361447, BL0361446 |
| Therapeutic Target Database | T76685 |
| ChEMBL | CHEMBL218 |
| IUPHAR | 56 |
| DrugBank | BE0000061 |
| Name | Rimonabant |
|---|---|
| Molecular formula | C22H21Cl3N4O |
| IUPAC name | 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-piperidin-1-ylpyrazole-3-carboxamide |
| Molecular weight | 463.787 |
| Hydrogen bond acceptor | 3 |
| Hydrogen bond donor | 1 |
| XlogP | 6.5 |
| Synonyms | KB-12498 1H-Pyrazole-3-carboxamide, 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-1-piperidinyl- Monaslim (TN) 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide 81R131 [ Show all ] |
| Inchi Key | JZCPYUJPEARBJL-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) |
| PubChem CID | 104850 |
| ChEMBL | CHEMBL111 |
| IUPHAR | 743 |
| BindingDB | 21278 |
| DrugBank | DB06155 |
Structure | 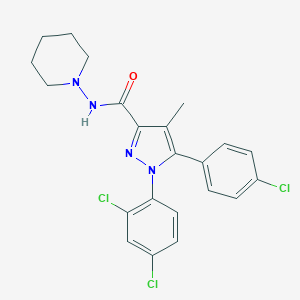 |
| Lipinski's druglikeness | This ligand has a partition coefficient log P greater than 5. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| N/A | N/A | DrugBank | |
| Activity | 13.15 pmol | PMID18585913 | ChEMBL |
| Bmax | 1.7 pmol/mg | PMID17004712 | ChEMBL |
| Decrease | -85.0 % | PMID15801840 | ChEMBL |
| Displacement | 46.0 % | PMID14613317 | ChEMBL |
| EC50 | 0.11 nM | PMID20047779 | BindingDB,ChEMBL |
| EC50 | 1.5 nM | PMID24175572 | ChEMBL |
| EC50 | 1.6 nM | PMID19351113 | BindingDB |
| EC50 | 4.0 nM | PMID19520572, PMID18083560 | BindingDB,ChEMBL |
| EC50 | 5.0 nM | PMID26827137 | ChEMBL |
| EC50 | 5.0 nM | PMID26827137 | BindingDB |
| EC50 | 10.1 nM | PMID16279809, PMID16451053 | BindingDB,ChEMBL |
| EC50 | 15.7 nM | PMID19530697 | BindingDB,ChEMBL |
| EC50 | 18.2 nM | PMID19095444, PMID18712856 | BindingDB,ChEMBL |
| EC50 | 50.0 nM | PMID25096297 | BindingDB,ChEMBL |
| EC50 | 50.12 nM | PMID25096297 | ChEMBL |
| EC50 | 240.0 nM | PMID19328683 | BindingDB,ChEMBL |
| Efficacy | 0.0 % | PMID24900561 | ChEMBL |
| Emax | -84.0 % | PMID16279809 | ChEMBL |
| Emax | 98.89 % | PMID25096297 | ChEMBL |
| IC50 | 1.35 nM | PMID18448340 | BindingDB,ChEMBL |
| IC50 | 2.1 nM | PMID20005704 | BindingDB,ChEMBL |
| IC50 | 2.2 nM | PMID18754613 | BindingDB,ChEMBL |
| IC50 | 2.9 nM | PMID20015647 | BindingDB,ChEMBL |
| IC50 | 3.0 nM | PMID17433696 | BindingDB,ChEMBL |
| IC50 | 3.2 nM | PMID20047779 | BindingDB,ChEMBL |
| IC50 | 4.0 nM | PMID21376588 | BindingDB,ChEMBL |
| IC50 | 4.5 nM | PMID19954978, PMID20015647 | BindingDB,ChEMBL |
| IC50 | 5.1 nM | PMID20015647 | BindingDB,ChEMBL |
| IC50 | 6.0 nM | PMID15713403 | BindingDB,ChEMBL |
| IC50 | 6.1 nM | PMID17181138, PMID17293109 | BindingDB,ChEMBL |
| IC50 | 6.2 nM | PMID15664830 | BindingDB,ChEMBL |
| IC50 | 11.22 nM | PMID21334892 | BindingDB,ChEMBL |
| IC50 | 13.0 nM | PMID25644673, PMID24445310 | BindingDB,ChEMBL |
| IC50 | 13.2 nM | PMID19530697 | BindingDB,ChEMBL |
| IC50 | 13.5 nM | PMID26151231 | ChEMBL |
| IC50 | 14.0 nM | PMID26151231 | BindingDB |
| IC50 | 15.0 nM | PMID19095444, PMID18712856 | BindingDB,ChEMBL |
| IC50 | 51.0 nM | PMID22959249 | BindingDB,ChEMBL |
| IC50 | 63.1 nM | PMID19338356 | ChEMBL |
| IC50 | 108.0 nM | PMID20045337 | BindingDB,ChEMBL |
| IC50 | 120.0 nM | PMID18243711 | BindingDB,ChEMBL |
| Inhibition | 0.0 % | PMID22916707 | ChEMBL |
| Inhibition | 100.0 % | PMID24445310 | ChEMBL |
| Kb | 0.698 nM | PMID18800770 | ChEMBL |
| Kd | 1.8 nM | PMID17004712 | BindingDB,ChEMBL |
| Kd | 2.1 nM | PMID24092756 | BindingDB |
| Kd | 2.512 nM | PMID16140010, PMID20363132, PMID14736243, PMID15771428 | ChEMBL |
| Kd | 2.57 nM | PMID20845959, PMID18512901 | ChEMBL |
| Ke | 1.1 nM | PMID26827137, PMID22372835, PMID24944734, PMID20845959 | ChEMBL |
| Ki | 0.19 nM | PMID19102698 | ChEMBL |
| Ki | 0.19 nM | PMID19102698 | PDSP |
| Ki | 0.4 nM | PMID18754613 | PDSP |
| Ki | 0.4 nM | PMID18754613 | BindingDB,ChEMBL |
| Ki | 0.43 nM | PMID18448340 | BindingDB,ChEMBL |
| Ki | 0.73 nM | PMID21962575 | BindingDB,ChEMBL |
| Ki | 0.74 nM | PMID20047779 | PDSP |
| Ki | 0.74 nM | PMID20047779 | BindingDB,ChEMBL |
| Ki | 0.9 nM | PMID19102698 | BindingDB,ChEMBL |
| Ki | 0.9 nM | PMID19102698 | PDSP |
| Ki | 0.933 nM | PMID21334892 | BindingDB |
| Ki | 0.9333 nM | PMID21334892 | ChEMBL |
| Ki | 1.0 nM | PMID16263283 | BindingDB,ChEMBL |
| Ki | 1.1 nM | PMID17383180 | BindingDB,ChEMBL |
| Ki | 1.1 nM | PMID17383180 | PDSP |
| Ki | 1.18 nM | PMID18512901 | PDSP |
| Ki | 1.18 nM | PMID20845959, PMID18512901 | BindingDB,ChEMBL |
| Ki | 1.38 nM | PMID19767206 | ChEMBL |
| Ki | 1.4 nM | PMID21428406, PMID19767206 | BindingDB,ChEMBL |
| Ki | 1.6 nM | PMID19683918, PMID19351113, PMID24900484 | BindingDB,ChEMBL |
| Ki | 1.6 nM | PMID19683918, PMID19351113 | PDSP |
| Ki | 1.8 nM | PMID18083560 | PDSP |
| Ki | 1.8 nM | PMID19351113, PMID18083560, PMID24900484 | BindingDB,ChEMBL |
| Ki | 1.9 nM | PMID19520572 | BindingDB,ChEMBL |
| Ki | 1.9 nM | PMID19520572 | PDSP |
| Ki | 1.98 nM | PMID18363352, PMID24445310 | ChEMBL |
| Ki | 1.99 - 12.6 nM | PMID7565624, PMID8819477, PMID9435190, PMID8070571, PMID12663689 | IUPHAR |
| Ki | 1.995 nM | PMID19338356 | ChEMBL |
| Ki | 2.0 nM | PMID19338356 | BindingDB |
| Ki | 2.1 nM | PMID16263283 | BindingDB,ChEMBL |
| Ki | 2.4 nM | PMID18363352, PMID20018510 | PDSP |
| Ki | 2.4 nM | PMID18363352, PMID20018510 | BindingDB,ChEMBL |
| Ki | 4.8 nM | PMID14613317 | BindingDB,ChEMBL |
| Ki | 5.37 nM | PMID16392793 | BindingDB,ChEMBL |
| Ki | 5.4 nM | PMID16279809, PMID16451053, PMID15801840 | BindingDB,ChEMBL |
| Ki | 5.6 nM | PMID19095444 | PDSP |
| Ki | 5.6 nM | PMID19095444 | BindingDB,ChEMBL |
| Ki | 5.9 nM | PMID24175572 | ChEMBL |
| Ki | 6.0 nM | PMID18335976 | PDSP,BindingDB,ChEMBL |
| Ki | 6.18 nM | PMID18512901 | PDSP |
| Ki | 6.18 nM | PMID20845959, PMID18512901 | BindingDB,ChEMBL |
| Ki | 6.2 nM | PMID22372835, PMID26827137 | BindingDB,ChEMBL |
| Ki | 7.1 nM | PMID17004712 | BindingDB,ChEMBL |
| Ki | 7.3 nM | PMID24936232, PMID23434135 | BindingDB,ChEMBL |
| Ki | 8.0 nM | PMID18579386 | PDSP,BindingDB,ChEMBL |
| Ki | 8.9 nM | PMID11960486, PMID10465552 | BindingDB,ChEMBL |
| Ki | 9.6 nM | PMID14613317 | BindingDB,ChEMBL |
| Ki | 10.6 nM | PMID23406429, PMID23072339 | BindingDB,ChEMBL |
| Ki | 11.0 nM | PMID23406429, PMID24729834 | BindingDB,ChEMBL |
| Ki | 12.0 nM | PMID18293908, PMID26756097, PMID19595596, PMID20943290, PMID14980654, PMID23085772, PMID21702498, PMID22548457, PMID20718492, PMID18680276 | PDSP,BindingDB,ChEMBL |
| Ki | 12.6 nM | PMID24900561, PMID22916707 | BindingDB,ChEMBL |
| Ki | 13.0 nM | PMID24900561 | BindingDB |
| Ki | 14.0 nM | PMID14613317 | BindingDB,ChEMBL |
| Ki | 16.0 nM | PMID17942307 | PDSP,BindingDB,ChEMBL |
| Ki | 18.0 nM | PMID17004712 | BindingDB,ChEMBL |
| Ki | 25.0 nM | PMID18342403, PMID20047331, PMID14736243, PMID16140010, PMID15771428, PMID20363132 | PDSP,BindingDB,ChEMBL |
| Ki | 33.0 nM | PMID14613317 | BindingDB,ChEMBL |
| Ki | 40.0 nM | PMID18511157 | PDSP,BindingDB,ChEMBL |
| Ki | 47.0 nM | PMID17979261 | PDSP,BindingDB,ChEMBL |
| Kieq | 12.0 nM | PMID26756097 | ChEMBL |
| Log Ki | 1.09 nM | PMID10882356 | ChEMBL |
zhanglab![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218