

You can:
| Name | Muscarinic acetylcholine receptor M1 |
|---|---|
| Species | Mus musculus (Mouse) |
| Gene | Chrm1 |
| Synonym | cholinergic receptor, muscarinic 1 cholinergic receptor, muscarinic 1, CNS cholinergic receptor M1 muscarinic acetylcholine receptor M1 receptor [ Show all ] |
| Disease | N/A for non-human GPCRs |
| Length | 460 |
| Amino acid sequence | MNTSVPPAVSPNITVLAPGKGPWQVAFIGITTGLLSLATVTGNLLVLISFKVNTELKTVNNYFLLSLACADLIIGTFSMNLYTTYLLMGHWALGTLACDLWLALDYVASNASVMNLLLISFDRYFSVTRPLSYRAKRTPRRAALMIGLAWLVSFVLWAPAILFWQYLVGERTVLAGQCYIQFLSQPIITFGTAMAAFYLPVTVMCTLYWRIYRETENRARELAALQGSETPGKGGGSSSSSERSQPGAEGSPESPPGRCCRCCRAPRLLQAYSWKEEEEEDEGSMESLTSSEGEEPGSEVVIKMPMVDPEAQAPTKQPPKSSPNTVKRPTKKGRDRGGKGQKPRGKEQLAKRKTFSLVKEKKAARTLSAILLAFILTWTPYNIMVLVSTFCKDCVPETLWELGYWLCYVNSTVNPMCYALCNKAFRDTFRLLLLCRWDKRRWRKIPKRPGSVHRTPSRQC |
| UniProt | P12657 |
| Protein Data Bank | N/A |
| GPCR-HGmod model | N/A |
| 3D structure model | No available structures or models |
| BioLiP | N/A |
| Therapeutic Target Database | N/A |
| ChEMBL | CHEMBL3733 |
| IUPHAR | 13 |
| DrugBank | N/A |
| Name | SCHEMBL8307259 |
|---|---|
| Molecular formula | C9H11F3N4O3 |
| IUPAC name | 3-methyl-5-(1,4,5,6-tetrahydropyrimidin-5-yl)-1,2,4-oxadiazole;2,2,2-trifluoroacetic acid |
| Molecular weight | 280.207 |
| Hydrogen bond acceptor | 9 |
| Hydrogen bond donor | 2 |
| XlogP | None |
| Synonyms | 1,4,5,6-Tetrahydro-5-(3-methyl-1,2,4-oxadiazol-5-yl)pyrimidine trifluoroacetate VNNPKITYMMVTIG-UHFFFAOYSA-N |
| Inchi Key | VNNPKITYMMVTIG-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C7H10N4O.C2HF3O2/c1-5-10-7(12-11-5)6-2-8-4-9-3-6;3-2(4,5)1(6)7/h4,6H,2-3H2,1H3,(H,8,9);(H,6,7) |
| PubChem CID | 9838580 |
| ChEMBL | CHEMBL277014 |
| IUPHAR | N/A |
| BindingDB | N/A |
| DrugBank | N/A |
Structure | 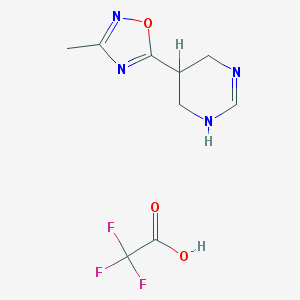 |
| Lipinski's druglikeness | Partition coefficient log P of this ligand is not available. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| EC50 | 5400.0 nM | PMID9111297 | ChEMBL |
| Inhibition | 30.9 % | PMID9111297 | ChEMBL |
| Inhibition | 69.1 % | PMID9111297 | ChEMBL |
| Inhibition | 98.3 % | PMID9111297 | ChEMBL |
| Ki | 2.31 nM | PMID9111297 | ChEMBL |
| Ki | 2.5 nM | PMID9111297 | ChEMBL |
| Ki | 21.1 nM | PMID9111297 | ChEMBL |
| Smax | 640.0 % | PMID9111297 | ChEMBL |
zhanglab![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218