

You can:
| Name | Delta-type opioid receptor |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | OPRD1 |
| Synonym | D-OR-1 DOR opioid receptor OP1 DOP [ Show all ] |
| Disease | Cough Overactive bladder disorder Bladder disease Moderate-to-severe pain Diarrhea-predominant IBS [ Show all ] |
| Length | 372 |
| Amino acid sequence | MEPAPSAGAELQPPLFANASDAYPSACPSAGANASGPPGARSASSLALAIAITALYSAVCAVGLLGNVLVMFGIVRYTKMKTATNIYIFNLALADALATSTLPFQSAKYLMETWPFGELLCKAVLSIDYYNMFTSIFTLTMMSVDRYIAVCHPVKALDFRTPAKAKLINICIWVLASGVGVPIMVMAVTRPRDGAVVCMLQFPSPSWYWDTVTKICVFLFAFVVPILIITVCYGLMLLRLRSVRLLSGSKEKDRSLRRITRMVLVVVGAFVVCWAPIHIFVIVWTLVDIDRRDPLVVAALHLCIALGYANSSLNPVLYAFLDENFKRCFRQLCRKPCGRPDPSSFSRAREATARERVTACTPSDGPGGGAAA |
| UniProt | P41143 |
| Protein Data Bank | 4rwd, 4rwa, 4n6h |
| GPCR-HGmod model | P41143 |
| 3D structure model | This structure is from PDB ID 4rwd. |
| BioLiP | BL0303696,BL0303697, BL0265712, BL0265705,BL0265706,BL0265707,, BL0303699,BL0303701, BL0303698,BL0303700 |
| Therapeutic Target Database | T58992 |
| ChEMBL | CHEMBL236 |
| IUPHAR | 317 |
| DrugBank | BE0000420 |
| Name | Naltrindole |
|---|---|
| Molecular formula | C26H26N2O3 |
| IUPAC name | (1S,2S,13R,21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazaheptacyclo[13.9.1.01,13.02,21.04,12.05,10.019,25]pentacosa-4(12),5,7,9,15,17,19(25)-heptaene-2,16-diol |
| Molecular weight | 414.505 |
| Hydrogen bond acceptor | 4 |
| Hydrogen bond donor | 3 |
| XlogP | 3.3 |
| Synonyms | 4,8-methanobenzofuro[2,3-a]pyrido[4,3-b]carbazole-1,8a(9H)-diol, 7-(cyclopropylmethyl)-5,6,7,8,14,14b-hexahydro-, (4bS,8R,8aS,14bR)- HMS2089E12 [3H]-naltrindole D0M2EX NTI [ Show all ] |
| Inchi Key | WIYUZYBFCWCCQJ-IFKAHUTRSA-N |
| Inchi ID | InChI=1S/C26H26N2O3/c29-19-8-7-15-11-20-26(30)12-17-16-3-1-2-4-18(16)27-22(17)24-25(26,21(15)23(19)31-24)9-10-28(20)13-14-5-6-14/h1-4,7-8,14,20,24,27,29-30H,5-6,9-13H2/t20-,24+,25+,26-/m1/s1 |
| PubChem CID | 5497186 |
| ChEMBL | CHEMBL567175 |
| IUPHAR | 1641, 3829 |
| BindingDB | 50370067 |
| DrugBank | N/A |
Structure | 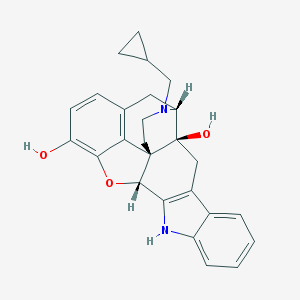 |
| Lipinski's druglikeness | This ligand satisfies Lipinski's rule of five. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| Activity | -3.0 % | PMID18637671 | ChEMBL |
| Activity | 27.0 - | PMID7515442 | ChEMBL |
| Activity | 100.0 % | PMID21115248 | ChEMBL |
| EC50 | 0.11 Ke nM-1 | PMID12672258 | ChEMBL |
| EC50 | 0.11 nM | PMID12825957 | ChEMBL |
| ED50 ratio | 6.7 - | PMID7853332 | ChEMBL |
| Emax | -0.47 % | PMID19282177 | ChEMBL |
| IC50 | 0.32 nM | PMID1335078 | ChEMBL |
| IC50 | 0.39 nM | PMID19282177 | ChEMBL |
| IC50 | 0.91 nM | PMID23403082 | ChEMBL |
| IC50 ratio | 7.8 - | PMID9651172 | ChEMBL |
| IC50 ratio | 152.0 - | PMID1851846 | ChEMBL |
| Imax | 84.0 % | PMID19282177 | ChEMBL |
| Inhibition | 18.0 % | PMID23403082 | ChEMBL |
| Kd | 11.22 nM | PMID17266203 | ChEMBL |
| Ke | 0.11 nM | PMID24973818, PMID15588100, PMID15456250, PMID11784158 | ChEMBL |
| Ke | 0.18 nM | PMID17625813 | ChEMBL |
| Ke | 0.18 uM | PMID19053757 | ChEMBL |
| Ke | 0.21 nM | PMID14711299, PMID16942033 | ChEMBL |
| Ki | 0.03 nM | PMID7853332, PMID2160538, PMID1333013 | ChEMBL |
| Ki | 0.031 nM | PMID1648136 | ChEMBL |
| Ki | 0.04 nM | PMID12565965 | ChEMBL |
| Ki | 0.062 nM | PMID14998329, PMID12431065, PMID10229636 | ChEMBL |
| Ki | 0.07 nM | PMID14711299 | ChEMBL |
| Ki | 0.1072 nM | PMID17490886 | ChEMBL |
| Ki | 0.14 nM | PMID19282177 | ChEMBL |
| Ki | 0.15 nM | PMID11294396 | ChEMBL |
| Ki | 0.16 nM | PMID23403082, PMID12930147 | ChEMBL |
| Ki | 0.199526 nM | PMID9686407, PMID2832195 | IUPHAR |
| Ki | 0.2 nM | PMID12672258, PMID15588100, PMID12825957, PMID11784158, PMID15456250, PMID24973818 | ChEMBL |
| Ki | 0.2 nM | PMID24973818 | BindingDB |
| Ki | 0.22 nM | PMID11597422 | ChEMBL |
| Ki | 0.24 nM | PMID12930147 | ChEMBL |
| Ki | 0.31 - | PMID1851846 | ChEMBL |
| Ki | 0.457 nM | PMID26048798 | ChEMBL |
| Ki | 0.457 nM | PMID26048798 | BindingDB |
| Ki | 0.46 nM | PMID21621410 | ChEMBL |
| Ki | 0.5 nM | PMID21621410 | ChEMBL |
| Ki | 0.9 nM | PMID25193297 | BindingDB |
| Ki | 0.9 nM | PMID25193297, PMID23587424 | ChEMBL |
| Ki | 1.97 nM | PMID19595591 | ChEMBL |
| Ratio | 2.6 - | PMID7853332 | ChEMBL |
| Ratio | 76.0 - | PMID9301674 | ChEMBL |
| Ratio | 152.0 - | PMID8126697 | ChEMBL |
| Ratio | 11066.0 - | PMID1333013 | ChEMBL |
| Selectivity ratio | 88.0 - | PMID9667975 | ChEMBL |
| Selectivity ratio | 153.0 - | PMID9207938 | ChEMBL |
| Selectivity ratio | 8375.0 - | PMID9207938 | ChEMBL |
zhanglab![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218