

You can:
| Name | Free fatty acid receptor 1 |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | FFAR1 |
| Synonym | FFA1R G protein-coupled receptor 40 G-protein coupled receptor 40 GPR40 FFA1 receptor |
| Disease | Type 2 diabetes Non-insulin dependent diabetes Diabetes |
| Length | 300 |
| Amino acid sequence | MDLPPQLSFGLYVAAFALGFPLNVLAIRGATAHARLRLTPSLVYALNLGCSDLLLTVSLPLKAVEALASGAWPLPASLCPVFAVAHFFPLYAGGGFLAALSAGRYLGAAFPLGYQAFRRPCYSWGVCAAIWALVLCHLGLVFGLEAPGGWLDHSNTSLGINTPVNGSPVCLEAWDPASAGPARFSLSLLLFFLPLAITAFCYVGCLRALARSGLTHRRKLRAAWVAGGALLTLLLCVGPYNASNVASFLYPNLGGSWRKLGLITGAWSVVLNPLVTGYLGRGPGLKTVCAARTQGGKSQK |
| UniProt | O14842 |
| Protein Data Bank | 5tzy, 5tzr |
| GPCR-HGmod model | O14842 |
| 3D structure model | This structure is from PDB ID 5tzy. |
| BioLiP | BL0380462, BL0380463, BL0380464 |
| Therapeutic Target Database | T25608 |
| ChEMBL | CHEMBL4422 |
| IUPHAR | 225 |
| DrugBank | BE0000688 |
| Name | GW9508 |
|---|---|
| Molecular formula | C22H21NO3 |
| IUPAC name | 3-[4-[(3-phenoxyphenyl)methylamino]phenyl]propanoic acid |
| Molecular weight | 347.414 |
| Hydrogen bond acceptor | 4 |
| Hydrogen bond donor | 2 |
| XlogP | 4.7 |
| Synonyms | 3-(4-((3-Phenoxybenzyl)amino)phenyl)propanoic acid 885101-89-3 AX8208187 CCG-221855 EX-A223 [ Show all ] |
| Inchi Key | DGENZVKCTGIDRZ-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C22H21NO3/c24-22(25)14-11-17-9-12-19(13-10-17)23-16-18-5-4-8-21(15-18)26-20-6-2-1-3-7-20/h1-10,12-13,15,23H,11,14,16H2,(H,24,25) |
| PubChem CID | 11595431 |
| ChEMBL | CHEMBL207881 |
| IUPHAR | 1050 |
| BindingDB | 22496 |
| DrugBank | N/A |
Structure | 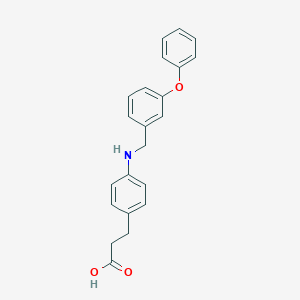 |
| Lipinski's druglikeness | This ligand satisfies Lipinski's rule of five. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| %max | 69.0 % | PMID17240142 | ChEMBL |
| %max | 82.0 % | PMID16439116 | ChEMBL |
| Activity | 223.0 nM | PMID17552505 | ChEMBL |
| EC50 | <30000.0 nM | PMID17552505 | BindingDB,ChEMBL |
| EC50 | 13.0 nM | PMID24835985 | BindingDB,ChEMBL |
| EC50 | 28.0 nM | PMID27074625 | BindingDB |
| EC50 | 28.18 nM | PMID22519963, PMID27074625 | BindingDB,ChEMBL |
| EC50 | 28.84 nM | PMID24900217 | ChEMBL |
| EC50 | 47.86 nM | PMID18947221, PMID21854074 | BindingDB,ChEMBL |
| EC50 | 50.1187 nM | PMID16702987 | IUPHAR |
| EC50 | 64.57 nM | PMID16439116 | ChEMBL |
| EC50 | 79.0 nM | PMID17240142 | BindingDB |
| EC50 | 79.43 nM | PMID17240142 | ChEMBL |
| EC50 | 87.1 nM | PMID24900217 | ChEMBL |
| EC50 | 100.0 nM | PMID20064714 | BindingDB,ChEMBL |
| EC50 | 223.87 nM | PMID17552505 | ChEMBL |
| EC50 | 316.0 nM | PMID25441945 | BindingDB |
| EC50 | 316.23 nM | PMID25441945 | ChEMBL |
| EC50 | 1200.0 nM | PMID17552505 | BindingDB,ChEMBL |
| EC50 | 2200.0 nM | PMID17552505 | BindingDB,ChEMBL |
| EC50 | 6800.0 nM | PMID17552505 | BindingDB,ChEMBL |
| EC50 | 17800.0 nM | PMID17552505 | BindingDB,ChEMBL |
| Efficacy | 86.0 % | PMID22519963 | ChEMBL |
| Efficacy | 103.0 % | PMID24900217 | ChEMBL |
| Emax | 86.0 % | PMID27074625 | ChEMBL |
| Fold change | 5.39 - | PMID17552505 | ChEMBL |
| Fold change | 6.48 - | PMID17552505 | ChEMBL |
| Fold change | 7.35 - | PMID17552505 | ChEMBL |
| Ki | 218.78 nM | PMID27074625 | ChEMBL |
| Ki | 219.0 nM | PMID27074625 | BindingDB |
zhanglab![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218