

You can:
| Name | Adenosine receptor A1 |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | ADORA1 |
| Synonym | RDC7 A1 receptor A1-AR A1R adenosine receptor A1 |
| Disease | Cardiac arrhythmias Hypertension Cardiac disease Cognitive disorders Diabetes [ Show all ] |
| Length | 326 |
| Amino acid sequence | MPPSISAFQAAYIGIEVLIALVSVPGNVLVIWAVKVNQALRDATFCFIVSLAVADVAVGALVIPLAILINIGPQTYFHTCLMVACPVLILTQSSILALLAIAVDRYLRVKIPLRYKMVVTPRRAAVAIAGCWILSFVVGLTPMFGWNNLSAVERAWAANGSMGEPVIKCEFEKVISMEYMVYFNFFVWVLPPLLLMVLIYLEVFYLIRKQLNKKVSASSGDPQKYYGKELKIAKSLALILFLFALSWLPLHILNCITLFCPSCHKPSILTYIAIFLTHGNSAMNPIVYAFRIQKFRVTFLKIWNDHFRCQPAPPIDEDLPEERPDD |
| UniProt | P30542 |
| Protein Data Bank | 6d9h, 5n2s |
| GPCR-HGmod model | P30542 |
| 3D structure model | This structure is from PDB ID 6d9h. |
| BioLiP | BL0385576, BL0417675 |
| Therapeutic Target Database | T88714, T92072 |
| ChEMBL | CHEMBL226 |
| IUPHAR | 18 |
| DrugBank | BE0000013 |
| Name | 8-Cyclopentyl-1,3-dipropylxanthine |
|---|---|
| Molecular formula | C16H24N4O2 |
| IUPAC name | 8-cyclopentyl-1,3-dipropyl-7H-purine-2,6-dione |
| Molecular weight | 304.394 |
| Hydrogen bond acceptor | 3 |
| Hydrogen bond donor | 1 |
| XlogP | 4.0 |
| Synonyms | NCGC00023294-07 AKOS024458134 PD116948 BSPBio_002686 SCHEMBL382422 [ Show all ] |
| Inchi Key | FFBDFADSZUINTG-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) |
| PubChem CID | 1329 |
| ChEMBL | CHEMBL183 |
| IUPHAR | 386 |
| BindingDB | 21173 |
| DrugBank | N/A |
Structure | 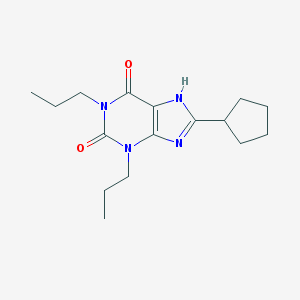 |
| Lipinski's druglikeness | This ligand satisfies Lipinski's rule of five. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| Activity | 45.0 % | PMID19836950 | ChEMBL |
| Activity | 93.0 % | MedChemComm, (2012) 3:3:333 | ChEMBL |
| Activity | 133.0 % | PMID18258439 | ChEMBL |
| Change in cAMP | 100.0 % | PMID15771447 | ChEMBL |
| IC50 | 0.5 nM | PMID26988801, PMID27876250 | ChEMBL |
| IC50 | 0.87 nM | PMID23582449 | ChEMBL |
| IC50 | 0.89 nM | PMID23466604 | ChEMBL |
| IC50 | 5.0 nM | PMID26988801, PMID27876250 | BindingDB |
| IC50 | 6.7 nM | PMID18983139 | ChEMBL |
| IC50 | 13.0 nM | PMID10476879, PMID18588282 | BindingDB,ChEMBL |
| IC50 | 14.0 nM | PMID10476879 | BindingDB,ChEMBL |
| IC50 | 25.0 nM | PMID20875743 | BindingDB,ChEMBL |
| Kd | 2.704 nM | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| Kd | 3.0 nM | PMID27282729 | BindingDB |
| Kd | 3.715 nM | PMID9871584 | ChEMBL |
| Ki | 0.47 nM | PMID1694546 | BindingDB,ChEMBL |
| Ki | 0.5 nM | PMID17927167, PMID24077183, PMID18269230, PMID22257095, PMID27282729, PMID16366607 | BindingDB,ChEMBL |
| Ki | 0.56 nM | PMID23466604 | ChEMBL |
| Ki | 0.63 - 39.8 nM | PMID9920910, PMID16020631, PMID15740718, PMID16902942, PMID8032613 | IUPHAR |
| Ki | 0.9 nM | PMID16759111, PMID20537438 | BindingDB,ChEMBL |
| Ki | 1.6 nM | PMID18307292, PMID18307293, PMID18189346 | BindingDB,ChEMBL |
| Ki | 2.0 nM | PMID11809867 | PDSP |
| Ki | 2.13 nM | PMID11960496 | BindingDB,ChEMBL |
| Ki | 2.2 nM | PMID26824742 | BindingDB,ChEMBL |
| Ki | 2.52 nM | PMID9191953 | ChEMBL |
| Ki | 2.93 nM | PMID9191953 | BindingDB |
| Ki | 3.0 nM | PMID17201410 | BindingDB,ChEMBL |
| Ki | 3.2 nM | PMID19301821, PMID20937560, PMID17665891, PMID15214785, PMID25462223, PMID16789747, PMID18468446, PMID16335918 | BindingDB,ChEMBL |
| Ki | 3.9 nM | PMID20408530 | BindingDB,ChEMBL |
| Ki | 4.0 nM | PMID19282184 | BindingDB,ChEMBL |
| Ki | 4.2 nM | PMID18983139 | ChEMBL |
| Ki | 6.1 nM | PMID18258439, J Med Chem. 2005 Mar 24;48(6):2045-53., PMID18637670, PMID15771447 | PDSP,BindingDB,ChEMBL |
| Ki | 6.5 nM | PMID16250647 | BindingDB,ChEMBL |
| Ki | 6.7 nM | PMID15163184 | BindingDB,ChEMBL |
| Ki | 7.0 nM | PMID10476879 | BindingDB,ChEMBL |
| Ki | 12.0 nM | PMID14736246 | BindingDB,ChEMBL |
| Ki | 12.02 nM | MedChemComm, (2012) 3:3:333 | ChEMBL |
| Ki | 12.9 nM | PMID19836950 | BindingDB,ChEMBL |
| Ki | 95.0 nM | PMID19036477 | BindingDB,ChEMBL |
| koff | 0.25 min^-1 | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| kon | 0.14 nM^-1 min^-1 | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| kon | 345900.0 Ms-1 | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| k_off | 0.0009352 s-1 | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| Production | 157.0 % | PMID11960496 | ChEMBL |
zhanglab![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218