

You can:
| Name | Muscarinic acetylcholine receptor M1 |
|---|---|
| Species | Rattus norvegicus (Rat) |
| Gene | Chrm1 |
| Synonym | cholinergic receptor, muscarinic 1 cholinergic receptor, muscarinic 1, CNS cholinergic receptor M1 muscarinic acetylcholine receptor M1 receptor [ Show all ] |
| Disease | N/A for non-human GPCRs |
| Length | 460 |
| Amino acid sequence | MNTSVPPAVSPNITVLAPGKGPWQVAFIGITTGLLSLATVTGNLLVLISFKVNTELKTVNNYFLLSLACADLIIGTFSMNLYTTYLLMGHWALGTLACDLWLALDYVASNASVMNLLLISFDRYFSVTRPLSYRAKRTPRRAALMIGLAWLVSFVLWAPAILFWQYLVGERTVLAGQCYIQFLSQPIITFGTAMAAFYLPVTVMCTLYWRIYRETENRARELAALQGSETPGKGGGSSSSSERSQPGAEGSPESPPGRCCRCCRAPRLLQAYSWKEEEEEDEGSMESLTSSEGEEPGSEVVIKMPMVDSEAQAPTKQPPKSSPNTVKRPTKKGRDRGGKGQKPRGKEQLAKRKTFSLVKEKKAARTLSAILLAFILTWTPYNIMVLVSTFCKDCVPETLWELGYWLCYVNSTVNPMCYALCNKAFRDTFRLLLLCRWDKRRWRKIPKRPGSVHRTPSRQC |
| UniProt | P08482 |
| Protein Data Bank | N/A |
| GPCR-HGmod model | N/A |
| 3D structure model | No available structures or models |
| BioLiP | N/A |
| Therapeutic Target Database | N/A |
| ChEMBL | CHEMBL276 |
| IUPHAR | 13 |
| DrugBank | N/A |
| Name | arecoline |
|---|---|
| Molecular formula | C8H13NO2 |
| IUPAC name | methyl 1-methyl-3,6-dihydro-2H-pyridine-5-carboxylate |
| Molecular weight | 155.197 |
| Hydrogen bond acceptor | 3 |
| Hydrogen bond donor | 0 |
| XlogP | 0.3 |
| Synonyms | Spectrum5_001316 DTXSID3022617 ZINC52541469 KBio2_000435 Lopac0_000049 [ Show all ] |
| Inchi Key | HJJPJSXJAXAIPN-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C8H13NO2/c1-9-5-3-4-7(6-9)8(10)11-2/h4H,3,5-6H2,1-2H3 |
| PubChem CID | 2230 |
| ChEMBL | CHEMBL7303 |
| IUPHAR | 296 |
| BindingDB | 46858 |
| DrugBank | DB04365 |
Structure | 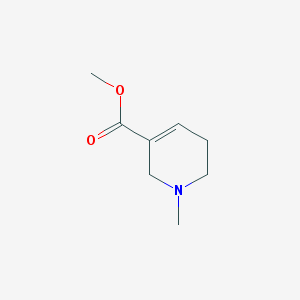 |
| Lipinski's druglikeness | This ligand satisfies Lipinski's rule of five. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| EC50 | 220.0 nM | PMID7990109 | ChEMBL |
| IC50 | 77.0 nM | PMID7658434, PMID1613751, PMID1433209 | BindingDB,ChEMBL |
| IC50 | 1100.0 nM | PMID3385727 | BindingDB,ChEMBL |
| IC50 | 1300.0 nM | , PMID7658434, PMID1433209, PMID1613751, Bioorg. Med. Chem. Lett., (1992) 2:8:809 | BindingDB,ChEMBL |
| IC50 | 1737.8 nM | Bioorg. Med. Chem. Lett., (1992) 2:5:501 | ChEMBL |
| IC50 | 1738.0 nM | N/A | BindingDB |
| IC50 | 1740.0 nM | PMID1732522 | BindingDB,ChEMBL |
| IC50 | 11920.0 nM | , Bioorg. Med. Chem. Lett., (1991) 1:3:147 | BindingDB,ChEMBL |
| IC50 | 460000.0 nM | PMID19595599 | BindingDB,ChEMBL |
| IC50 | 469000.0 nM | PMID19717214, PMID18359231 | BindingDB,ChEMBL |
| Inhibition | 13.0 % | PMID9622546 | ChEMBL |
| Inhibition | 43.0 % | PMID9622546 | ChEMBL |
| Ki | 5011.87 nM | PMID2537406 | IUPHAR |
| Ki | 5950.0 nM | PMID1310135 | BindingDB |
| Ki | 86000.0 nM | PMID19717214, PMID18359231 | BindingDB,ChEMBL |
| Ki | 88000.0 nM | PMID19595599 | BindingDB,ChEMBL |
| pD2 | 6.6 - | PMID1732522 | ChEMBL |
| Ratio | 87.0 - | PMID1732522 | ChEMBL |
zhanglab![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218