

You can:
| Name | Gastrin/cholecystokinin type B receptor |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | CCKBR |
| Synonym | CCK-B receptor CCK-B receptor {ECO:0000303|PubMed:8415658} CCK-B/gastrin receptor CCK-BR gastrin/cholecystokinin type B receptor [ Show all ] |
| Disease | Diagnostic imaging Duodenal ulcers Gastroesophageal reflux disease Gastrointestinal disease Intestine cancer [ Show all ] |
| Length | 447 |
| Amino acid sequence | MELLKLNRSVQGTGPGPGASLCRPGAPLLNSSSVGNLSCEPPRIRGAGTRELELAIRITLYAVIFLMSVGGNMLIIVVLGLSRRLRTVTNAFLLSLAVSDLLLAVACMPFTLLPNLMGTFIFGTVICKAVSYLMGVSVSVSTLSLVAIALERYSAICRPLQARVWQTRSHAARVIVATWLLSGLLMVPYPVYTVVQPVGPRVLQCVHRWPSARVRQTWSVLLLLLLFFIPGVVMAVAYGLISRELYLGLRFDGDSDSDSQSRVRNQGGLPGAVHQNGRCRPETGAVGEDSDGCYVQLPRSRPALELTALTAPGPGSGSRPTQAKLLAKKRVVRMLLVIVVLFFLCWLPVYSANTWRAFDGPGAHRALSGAPISFIHLLSYASACVNPLVYCFMHRRFRQACLETCARCCPRPPRARPRALPDEDPPTPSIASLSRLSYTTISTLGPG |
| UniProt | P32239 |
| Protein Data Bank | N/A |
| GPCR-HGmod model | P32239 |
| 3D structure model | This predicted structure model is from GPCR-EXP P32239. |
| BioLiP | N/A |
| Therapeutic Target Database | T05849 |
| ChEMBL | CHEMBL298 |
| IUPHAR | 77 |
| DrugBank | BE0001158 |
| Name | CHEMBL502398 |
|---|---|
| Molecular formula | C56H67N9O12 |
| IUPAC name | N-[6-[[2-[2-[[(4R,4aS,7S,7aR,12bS)-4a,10-dihydroxy-3-methyl-1,2,4,5,6,7,7a,13-octahydro-4,12-methanobenzofuro[3,2-e]isoquinolin-7-yl]amino]-2-oxoethoxy]acetyl]amino]hexyl]-2-[2-[2-[3-[[(3R)-1-methyl-2-oxo-5-phenyl-3H-1,4-benzodiazepin-3-yl]carbamoylamino]phenoxy]ethylamino]-2-oxoethoxy]acetamide |
| Molecular weight | 1058.2 |
| Hydrogen bond acceptor | 14 |
| Hydrogen bond donor | 8 |
| XlogP | 3.2 |
| Synonyms | BDBM50265492 1-{1-Diglycoldiamide-6-[m-(R(+)-1)ureaphenoxy]ethane}-2-(N-oxymorphyldiglycoldiamide)hexane |
| Inchi Key | AWJDNWMDTDIGNU-NGEQUAPQSA-N |
| Inchi ID | InChI=1S/C56H67N9O12/c1-64-25-21-55-49-36-27-38(66)30-43(49)77-51(55)41(19-20-56(55,73)44(64)28-36)61-48(70)34-75-32-46(68)58-23-11-4-3-10-22-57-45(67)31-74-33-47(69)59-24-26-76-39-16-12-15-37(29-39)60-54(72)63-52-53(71)65(2)42-18-9-8-17-40(42)50(62-52)35-13-6-5-7-14-35/h5-9,12-18,27,29-30,41,44,51-52,66,73H,3-4,10-11,19-26,28,31-34H2,1-2H3,(H,57,67)(H,58,68)(H,59,69)(H,61,70)(H2,60,63,72)/t41-,44+,51-,52-,55-,56+/m0/s1 |
| PubChem CID | 44580900 |
| ChEMBL | CHEMBL502398 |
| IUPHAR | N/A |
| BindingDB | 50265492 |
| DrugBank | N/A |
Structure | 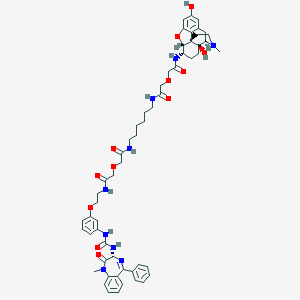 |
| Lipinski's druglikeness | This ligand has more than 5 hydrogen bond donor. This ligand has more than 10 hydrogen bond acceptor. This ligand is heavier than 500 daltons. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| Bmax | 9.3 pmol | PMID19113864 | ChEMBL |
| Bmax | 10.9 pmol | PMID19113864 | ChEMBL |
| Bmax | 18.5 pmol | PMID19113864 | ChEMBL |
| Ki | 16.3 nM | PMID19113864 | BindingDB,ChEMBL |
| Ki | 77.0 nM | PMID19113864 | BindingDB,ChEMBL |
| Ki | 260.0 nM | PMID19113864 | BindingDB,ChEMBL |
zhanglab![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218