
You can:
| Name | IB-MECA |
|---|---|
| Molecular formula | C18H19IN6O4 |
| IUPAC name | (2S,3S,4R,5R)-3,4-dihydroxy-5-[6-[(3-iodophenyl)methylamino]purin-9-yl]-N-methyloxolane-2-carboxamide |
| Molecular weight | 510.292 |
| Hydrogen bond acceptor | 8 |
| Hydrogen bond donor | 4 |
| XlogP | 0.9 |
| Synonyms | 215462-30-9 Adenosine, N(6)-[3-iodobenzyl]-4'-methylcarbamoyl-4'-dehydroxymethyl- BDBM50118812 CHEMBL119709 DTXSID50165158 [ Show all ] |
| Inchi Key | HUJXGQILHAUCCV-MOROJQBDSA-N |
| Inchi ID | InChI=1S/C18H19IN6O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1 |
| PubChem CID | 123683 |
| ChEMBL | CHEMBL119709 |
| IUPHAR | 422 |
| BindingDB | 50118812 |
| DrugBank | DB05511 |
Structure | 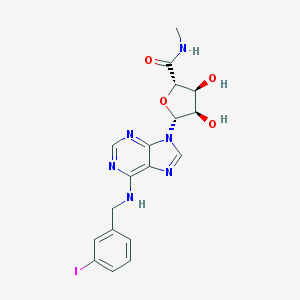 |
| Lipinski's druglikeness | This ligand is heavier than 500 daltons. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| EC50 | 79.43 nM | MedChemComm, (2014) 5:2:192 | ChEMBL |
| Ki | 32.0 nM | PMID9258366 | BindingDB,ChEMBL |
| Ki | 40.0 nM | PMID15743197 | BindingDB,ChEMBL |
| Ki | 56.0 nM | PMID15341491, PMID15481989 | BindingDB,ChEMBL |
| Ki | 93.0 nM | PMID18424135 | BindingDB |
| Ki | 93.1 nM | PMID18424135 | ChEMBL |
| Ki | 116.0 nM | PMID9572897 | BindingDB,ChEMBL |
| Ki | 143.0 nM | PMID9258366 | BindingDB,ChEMBL |
| Ki | 370.0 nM | PMID9258366 | BindingDB,ChEMBL |
| Ki | 501.187 - 2511.89 nM | PMID9459566, PMID7775460, PMID16518376 | IUPHAR |
| Ki | 510.0 nM | PMID16487705 | BindingDB,ChEMBL |
| Ki | 2520.0 nM | PMID10212124 | BindingDB,ChEMBL |
| Ki | 2900.0 nM | PMID15771421, PMID18811138, PMID19879151 | BindingDB,ChEMBL |
| Ki | 2910.0 nM | PMID15341938 | BindingDB,ChEMBL |
| Ki | 6200.0 nM | PMID27933810 | BindingDB,ChEMBL |
zhanglab![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218