

You can:
| Name | Sch 58261 |
|---|---|
| Molecular formula | C18H15N7O |
| IUPAC name | 4-(furan-2-yl)-10-(2-phenylethyl)-3,5,6,8,10,11-hexazatricyclo[7.3.0.02,6]dodeca-1(9),2,4,7,11-pentaen-7-amine |
| Molecular weight | 345.366 |
| Hydrogen bond acceptor | 6 |
| Hydrogen bond donor | 1 |
| XlogP | 2.4 |
| Synonyms | 4309023MAH AJ-08272 BDBM50048466 CS-5639 FT-0643554 [ Show all ] |
| Inchi Key | UTLPKQYUXOEJIL-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C18H15N7O/c19-18-22-16-13(11-20-24(16)9-8-12-5-2-1-3-6-12)17-21-15(23-25(17)18)14-7-4-10-26-14/h1-7,10-11H,8-9H2,(H2,19,22) |
| PubChem CID | 176408 |
| ChEMBL | CHEMBL17127 |
| IUPHAR | 431, 403 |
| BindingDB | 50048466 |
| DrugBank | N/A |
Structure | 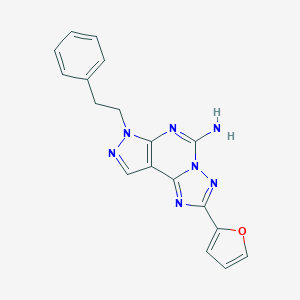 |
| Lipinski's druglikeness | This ligand satisfies Lipinski's rule of five. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| Activity | 0.25 pmol/ml | PMID20303771 | ChEMBL |
| Activity | 0.55 pmol/ml | PMID23953686 | ChEMBL |
| ED50 | 0.3 uM | PMID8676354 | ChEMBL |
| IC50 | 12.0 nM | PMID9622554 | BindingDB,ChEMBL |
| Kd | 1.0 - 2.51189 nM | PMID9933143, PMID9920286 | IUPHAR |
| Kd | 1.973 nM | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| Kd | 3.34 nM | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| Kd | 4.022 nM | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| Kd | 6.516 nM | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| Kd | 16.8 nM | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| Ki | 0.6 nM | PMID15149696 | BindingDB |
| Ki | 0.6 nM | PMID11809867, PMID23200243, PMID18637670, PMID9933143, PMID15149696 | PDSP,BindingDB,ChEMBL |
| Ki | 0.630957 - 5.01187 nM | PMID9933143, PMID9920286, PMID9179373 | IUPHAR |
| Ki | 1.1 nM | PMID22204739, PMID9622554, PMID24164628, PMID11754583, PMID19501513 | BindingDB,ChEMBL |
| Ki | 1.23 nM | PMID20303771 | BindingDB,ChEMBL |
| Ki | 1.9 nM | PMID23200243 | ChEMBL |
| Ki | 2.0 nM | PMID18562199, PMID18558486, PMID15163184 | BindingDB,ChEMBL |
| Ki | 2.3 nM | PMID16153830 | BindingDB,ChEMBL |
| Ki | 3.25 nM | PMID15163184 | BindingDB,ChEMBL |
| Ki | 4.3 nM | PMID15808481 | BindingDB,ChEMBL |
| koff | 0.4569 min^-1 | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| kon | 0.1278 nM^-1 min^-1 | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| kon | 1136000.0 Ms-1 | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| kon | 1480000.0 Ms-1 | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| kon | 2110000.0 Ms-1 | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| kon | 3528670.0 Ms-1 | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| kon | 3647870.0 Ms-1 | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| k_off | 0.003676 s-1 | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| k_off | 0.004112 s-1 | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| k_off | 0.00891 s-1 | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| k_off | 0.0211 s-1 | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| k_off | 0.02265 s-1 | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
zhanglab![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218