| Synonyms | Pylor
Lorantis
Roletra (TN)
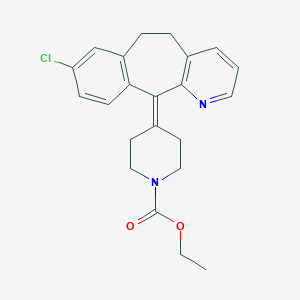
loratadine impurity c;4-(4,8-dichloro-5,6-dihydro-11h-benzo(5,6)cyclohepta(1,2-b)pyridin-11-ylidene)-1-piperidinecarboxylic acid ethyl ester
Sch29851
Loratadine, Pharmaceutical Secondary Standard; Certified Reference Material
Spectrum2_001584
Loraver
SR-01000075968-4
MCULE-5073177964
Tidilor (TN)
NCGC00015619-01
UNII-7AJO3BO7QN
NCGC00015619-09
NCGC00023125-06
NSC721075
Lisino
Clarityne Dy Repetabs
D06ABF
AB00053224-16
DTXSID2023224
ACT04775
ethyl 4-{13-chloro-4-azatricyclo[9.4.0.0;{3,8}]pentadeca-1(11),3(8),4,6,12,14-hexaen-2-ylidene}piperidine-1-carboxylate
Alavert, Claritin, Loratadine
FT-0627976
BBL010757
HMS3268M16
Bonalerg
HY-17043
CHEBI:6538
KBio2_007112
Claratyne Decongestant
Claritin reditab
11-[N-(ethoxycarbonyl)-4-piperidylidene]-8-chloro-6,11-dihydro-5H-benzo-[5,6]cyclohepta[1,2-b]pyridine
Restamine
Loratadina [Spanish]
Sanelor
Loratadine [USAN:BAN:INN]
Sinhistan Dy
Loratadine-d4
Spectrum5_001650
Loritine
STK574925
MLS000758260
Topcare childrens allergy relief
NCGC00015619-04
Wyeth Brand of Loratadine
NCGC00015619-12
NCGC00261365-01
L000667
Opera_ID_1868
Lopac0_000680
4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cycloheptal[1,2-b]pyridin-11-ylidene-1-piperidinecarboxylic acid ethyl ester
CPD000058255
7AJO3BO7QN
DivK1c_000792
AB0012486
ethyl 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)piperidine-1-carboxylate
AK-88666
FC1275
AllergyX (TN)
HMS2051G11
BCP9000858
HMS3654L17
BSPBio_002300
J10034
Children's Claritin
KBioSS_001976
Claritin
1-Piperidenecarboxylic acid, 4-(8-chloro-5,6-duhydro-11H-benzo [5,6]cyclohepta[1,2-b]-pyridin-11-ylidene)-, ethyl ester
Claritine (TN)
Loradif
Rinomex
Loratadine 0.1 mg/ml in Methanol
Sch 29851
Loratadine, British Pharmacopoeia (BP) Reference Standard
SPBio_001548
Loratidine
SR-01000075968-1
LS-114660
Tadine
MolPort-002-507-846
Tox21_301532
NCGC00015619-07
Zeos
NCGC00023125-04
NSC-721075
Lertamine
Pharmakon1600-01503712
4-(8-Chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene-1-piperidinecarboxylic acidethyl ester
CTK8G0633
8-Chloro-6,11-dihydro-11-(1-ethoxycarbonyl-4-piperidylidene)-5H-benzo[5,6]cyclohepta[1,2-b]pyridine
DSSTox_GSID_23224
AC-2086
ethyl 4-(8-chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidine-1-carboxylate
Alavert
Fristamin
Anhissen
HMS2235G23
BIDD:GT0198
HSDB 3578
CCG-100786
KBio2_001976
Claratyne
Claritin Hives Relief
1-Piperidinecarboxylic acid, 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-, ethyl ester
Clarityne
Q-100833
Lorastine
s1358
Loratadine Redidose
SCHEMBL4596
Loratadine, United States Pharmacopeia (USP) Reference Standard
Spectrum3_000740
Lorfast
SR-01000075968-6
MFCD00672869
TL8005389
NCGC00015619-02
Velodan
NCGC00015619-10
NCGC00023125-07
KS-1079
NSC758628
Lomilan (TN)
Clarityne-D
794L755
AB00053224_17
Ethyl 4-(8-chloro-5,6-dihydro-11H-benzo(5,6)cyclohepta(1,2-b)pyridin-11-ylidene)-1-piperidinecarboxylate
Aerotina
ethyl 4-{13-chloro-4-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8),4,6,12,14-hexaen-2-ylidene}piperidine-1-carboxylate
Alavert;Claritin
GTPL7216
BCP0726000007
HMS3371D13
BRD-K82795137-001-02-3
I14-0747
KBio3_001520
Clarinase
Claritin Reditabs
Loracert
Rhinase
SBI-0050659.P003
Loratadine [USAN:USP:INN:BAN]
SMR000058255
Loratadinum
Spectrum_001496
Lowadina
SW197416-3
MLS001148466
Tox21_110185
NCGC00015619-05
Z1741979837
NCGC00015619-13
NCI60_041473
L0223
Oprea1_027965
4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-1-piperidinecarboxylic acid ethyl ester
Cronopen
8-chloro-11-(1-ethoxycarbonyl-4-piperidylidene)-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine
DL-436
AB06849
Ethyl 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidine)-1-piperidinecarboxylate
AKOS005499513
Flonidan
Allertidin
HMS2090O18
BDBM22876
HMS3714E09
C22H23ClN2O2
JCCNYMKQOSZNPW-UHFFFAOYSA-N
Civeran
Klaritin
Claritin (TN)
1-Piperidenecarboxylic acid,6-duhydro-11H-benzo [5,6]cyclohepta[1,2-b]-pyridin-11-ylidene)-, ethyl ester
Clarityn
Loranox
Roletra
Loratadine for system suitability, European Pharmacopoeia (EP) Reference Standard
Sch-29851
Loratadine, European Pharmacopoeia (EP) Reference Standard
SPECTRUM1503712
Loratyne
SR-01000075968-3
M-5196
Talorat Dy
NC00036
Tox21_500680
NCGC00015619-08
ZINC537931
NCGC00023125-05
NSC-758628
Lesidas
Polaratyne
Clarityne (TN)
4-(8-chloro-5,6-dihydrobenzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)piperidine-1-carboxylic acid ethyl ester
D00364
AB00053224-15
DSSTox_RID_76931
AC1L1H3E
ethyl 4-{13-chloro-4-azatricyclo[9.4.0.0(3),]pentadeca-1(15),3,5,7,11,13-hexaen-2-ylidene}piperidine-1-carboxylate
Alavert (TN)
Fristamin (TN)
Bactimicina allergy
HMS3262G21
Biloina
HTS028367
CCG-39362
KBio2_004544
Claratyne Cold
Claritin Hives Relief Reditab
1-piperidinecarboxylic acid,4-(8-chloro-5,6-dihydro-11H-benzo(5,6)cyclohepta(1,2-b)pyridin-11-ylidene)-,ethyl ester
REGID_for_CID_3957
Loratadina
SAM001246987
Loratadine Wyeth Brand
Sensibit
Loratadine,(S)
Spectrum4_000177
Lorfast (TN)
ST2413201
MLS000069647
Tocris-1944
NCGC00015619-03
Versal
NCGC00015619-11
NCGC00255171-01
L 9664
Nularef
Lopac-L-9664
Clarium
79794-75-5
DB00455
AB00053224_18
Ethyl 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-1-piperidinecarboxylate
AJ-23376
EU-0100680
Alerpriv
Histaloran
BCP22338
HMS3393G11
BRD-K82795137-001-10-6
IDI1_000792
CHEMBL998
KBioGR_000693
Clarinase Reperabs
Claritine
4-(8-Chloro-5,6-dihydro-11H-benzo(5,6)cyclohepta(1,2-b)pyridin-11-ylidene)-1-piperidinecarboxylic Acid Ethyl Ester
Loradex
Rinolan (TN)
Loratadine (JAN/USAN/INN)
SC-13436
Loratadine, >=98% (HPLC), powder
Sohotin
Loratadinum [Latin]
SR-01000075968
LP00680
Symphoral (TN)
MLS001423984
Tox21_110185_1
NCGC00015619-06
ZB014352
NCGC00023125-02
NINDS_000792
Lergy
Optimin
4-(8-Chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene-1-piperidinecarboxylic acid ethyl ester
CS-0887
8-chloro-6,11-dihydro-11-(1- ethoxycarbonyl-4-piperidylidene)-5H-benzo[5,6]cyclohepta[1,2-b]pyridine
DSSTox_CID_3224
AB2000066
ethyl 4-(8-chloro-5,6-dihydrobenzo[1,2]cyclohepta[2,4-b]pyridin-11-ylidene)piperidine-1-carboxylate
Alarin
Flonidan (TN)
AN-14336
HMS2093I15
Bedix Loratadina
HMS502H14
CAS-79794-75-5
KBio1_000792
CL0028
KS-00000JOZ
Claritin D
1-Piperidinecarboxylic acid, 4-(8-chloro-5,6-dihydro-11H-benzo(5,6)cyclohepta(1,2-b)pyridin-11-ylidene)-, ethyl ester
Clarityn (TN) [ Show all ] |
|---|


![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218